Activated PI3K Delta Syndrome (APDS) is an underdiagnosed, genetic primary immunodeficiency
If you suspect your patient may have APDS, it’s time to test.
Activated PI3K Delta Syndrome (APDS) is a rare, genetic primary immunodeficiency
If you suspect your patient may have APDS, it’s time to test.
Learn About APDS
APDS Signs & Symptoms
Learn more about APDS at allaboutapds-hcp.com.
Revaluating Diagnosis
Since APDS shares many features of other immune disorders, patients with APDS may have a previous diagnosis. 5 APDS may be mistaken for common variable immune deficiency (CVID) or hyper IgM syndrome (HIGM), while hematologists may not realize their patient with refractory cytopenias or autoimmune lymphoproliferative syndrome (ALPS) actually has APDS. 5-7
Get a Definitive Diagnosis
Genetic testing can definitively diagnose APDS and other primary immunodeficiencies, which may have targeted treatment options and help identify patients for participation in clinical research. 6-8
A suboptimal response to treatment should raise suspicion of an IEI, like APDS11
Timeline of the most common pathologies seen in APDS 5,9,10*
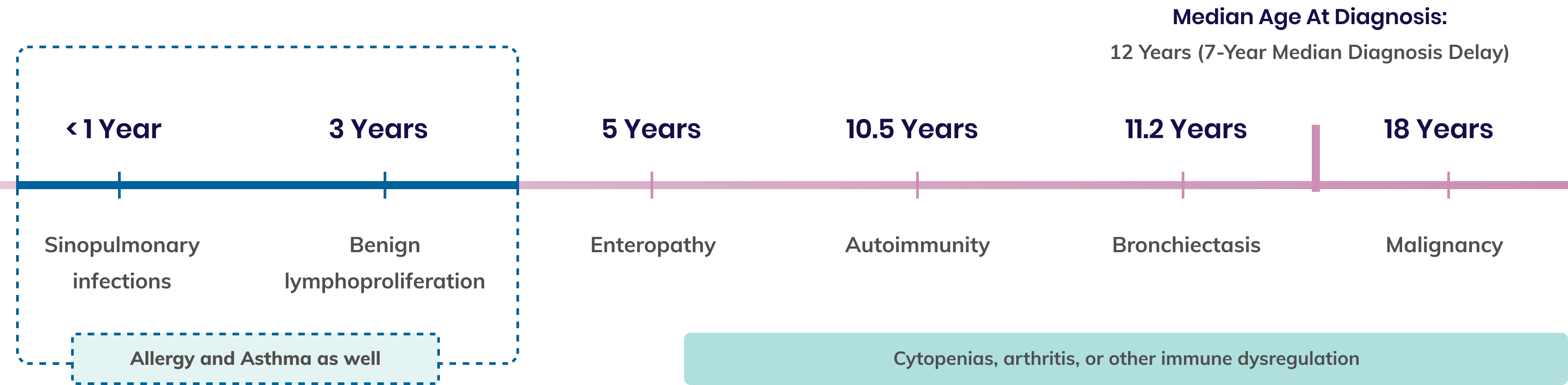
*Onset of all symptoms is heterogenous and typically progressive, varying from patient to patient
Definitively Diagnose APDS With Genetic Testing
The heterogenous signs and symptoms of APDS can often be attributed to other conditions, only leading to more questions. Once suspected, genetic testing can confirm the APDS diagnosis.
That’s why Pharming Healthcare, Inc. has partnered with Invitae (now part of Labcorp) to offer sponsored genetic testing and counseling for individuals who are suspected to have APDS.
Early, accurate diagnosis can change a person’s life and help get timely appropriate treatments.
Sponsored Program Eligibility
Sponsored genetic testing and genetic counseling are available for eligible patients regardless of insurance coverage or financial need.
Sponsored genetic testing and genetic counseling are available for eligible patients whether or not the patient has insurance or the patient’s insurance covers the test. Patients are not required to provide documentation that they are uninsured or that their insurance has denied coverage of the test.
Patients do not need to meet financial need criteria, such as having an income below a certain amount.
As long as the patient satisfies the clinical eligibility criteria to establish that the test is medically appropriate, the test and test results are available to the patient and his or her physician without any requirement whatsoever that the patient or physician take or withhold from any act based upon the test results they receive. This sponsored genetic testing program is provided so that patients and physicians can make better informed decisions about the patient’s appropriate course of treatment.
Testing Performed
APDS occurs when there is a disease-causing variant in either one of two specific genes, the PIK3CD gene or the PIK3R1 gene, in an autosomal dominant mode of inheritance. Healthcare professionals ordering tests for eligible patients under the sponsored testing program may select one of two genetic testing panels offered by Invitae to identify genetic variants associated with a broad range of inherited disorders of the immune system. Both panels include testing for the gene variants in PIK3CD or PIK3R1 that may indicate APDS. A positive test for either one of the two genes (PIK3CD or PIK3R1) may result in a definitive molecular diagnosis of APDS.
The Invitae Primary Immunodeficiency Panel analyzes genes that are associated with inherited disorders of the immune system. These genes were selected based on the available evidence to date and comprise Invitae’s broadest test for primary immunodeficiencies (PIDs).
The Invitae Inborn Errors of Immunity and Cytopenias Panel analyzes genes that are associated with inherited disorders of the immune system including inherited causes of cytopenia such as bone marrow failure and hereditary lymphoma. These genes were selected based on the available evidence to date and comprise Invitae’s broadest test for inborn errors of immunity.
Information Sharing and Outreach
Invitae will send the results of a test performed under the sponsored genetic testing program to the ordering healthcare professional.
Pharming Healthcare, Inc., as the sponsor of the genetic testing program, pays Invitae for and receives information about the number of tests performed under the program. For patients who are covered under commercial insurance or who do not have insurance, Pharming Healthcare also receives additional information for each test performed. Information provided to the testing program sponsor includes a random patient identification number assigned by the laboratory, the criteria that the patient met in order to be eligible for sponsored testing, certain patient demographic information such as age and sex, patient clinical history (including any family history of disease) provided on the test requisition form, information about the healthcare professional who ordered the test such as their name and contact information, and variants identified on the panel along with their classification. Information provided does not include the patient’s name, date of birth, or contact information.
If a patient tests positive for one of the disease-causing variants in PIK3CD or PIK3R1 that indicate APDS, Pharming Healthcare, Inc. may use the test-specific information received about anonymous uninsured patients and patients with commercial insurance to reach out to the ordering healthcare professional. The outreach may seek to provide the healthcare professional with additional information about APDS and a potential treatment option.
Some insurers may require that a patient test positive for one of the disease-causing variants (PIK3CD or PIK3R1) that indicate APDS before the insurer will approve coverage for targeted APDS treatment. Test results are not shared directly with insurers. However, the patient or their provider may choose to share test results with their insurer.
IMPORTANT CHANGE
The requisition form for ordering tests under the sponsored testing program now requires that the ordering healthcare professional indicate whether the patient is currently covered under a governmental insurance program.
Effective February 1, 2024, Pharming Healthcare, Inc. no longer receives ordering provider information for participating patients from the US, Canada, and Puerto Rico who are covered under governmental insurance programs such as Medicare and Medicaid. Completion of the new questionnaire allows Invitae to identify those patients to ensure the ordering provider information is no longer shared. This change does not affect patient eligibility. Patients covered under a governmental insurance program may still be eligible to receive testing and the results of that testing at no charge.
NavigateAPDS is a sponsored, genetic testing program available to individuals in the US, Canada, and Puerto Rico who meet any two of the bulleted criteria below:
Clinical Features
- Bronchiectasis
- Lymphadenopathy for greater than one month
- Chronic hepatomegaly or chronic splenomegaly
- Severe, persistent, or recurrent Herpesviridae
infections (e.g., EBV, cytomegalovirus) - Enteropathy
- Lymphoma at 0-25 years – meets the two eligibility criteria
- Lymphoma at ≥26 years of age – requires second eligibility criteria
Laboratory
- Elevated levels of immunoglobulin M
- Increased number of follicular helper T cells
- Reduced number of naïve B cells
History
- Common Variable Immune Deficiency (CVID)
phenotype or direct family member with CVID
phenotype - Relative with PIK3CD or PIK3R1 genotype (first
or second degree) – meets the two eligibility criteria
If you suspect APDS, and your patient meets the program criteria, testing is the next step.
Request free Specimen Collection Kits:
Individual or multiple kits can be ordered through the link below.
Blood kits (EDTA)

Buccal swab kits

Saliva kits

Sponsored Genetic Counseling Is Available
After Testing
Individuals tested through the navigateAPDS program are eligible for post-test genetic counseling to help them understand their test results. This service is provided through Genome Medical, an independent genetic counseling service, and is made available by Pharming Healthcare, Inc.
If requested, patients will be contacted by Genome Medical to schedule an appointment.
Visit the Genome Medical website to Learn More.
Book Genetic Counseling
Post-test genetic counseling is available as part of the navigateAPDS program.
To refer a patient for genetic counseling, physicians can check the box* under the section titled Genetic Counseling while completing the Test Requisition Form (TRF).
TRFs can be completed through
Invitae’s online portal (preferred) or the paper form.
*Checking this box will authorize the laboratory to share the necessary information with Genome Medical to complete the post-test genetic counseling.

Need Assistance Interpreting a Variant in PIK3CD/R1?
Genomenon’s Mastermind platform empowers HCPs by providing comprehensive evidence for interpretation of genetic test reports, which enables faster, more accurate diagnoses for patients.
Learn more about APDS at
References:
1. Activated PI3K-delta syndrome. Orphanet. Accessed April 26, 2021. https://www.orpha.net/consor/cgi-bin/OC_Exp.php?lng=EN&Expert=397596 2. Lucas CL, et al. Nat Immunol. 2014;15:88-97. 3. Angulo I, et al. Science. 2013;342(6160):866-871. 4. Lucas CL, et al. J Exp Med. 2014;211(13):2537-2547. 5. Jamee M, et al. Clin Rev Allergy Immunol. 2020;59(3):323-333 6. Rotz, SJ, et al. Pediatr Blood Cancer. 2018; 65:e27260. 7. Kulm E, et al. Oral abstract presented at the 62nd Annual ASH Meeting; Dec 5-8, 2020 8. Chinn IK, et al. J Allergy Clin Immunol. 2020;145(1):46-69. 9. Maccari ME et al. Front. Immunol. 2018;9:543 10. Elkaim E et al. J Allergy Clin Immunol. 2016;138(1):210-218. 11. Castagnoli R, et al. World Allergy Organ J. 2021;14(2):100513.
Pharming Healthcare, Inc. provides financial support for the genetic testing program and provides information about the program to healthcare professionals and others. Tests and services, however, are performed by Invitae. Healthcare professionals must confirm that patients meet certain criteria to use the program. Pharming Healthcare, Inc. and other third parties, including commercial organizations, may receive information about patients, the healthcare professionals ordering tests, and the test results, but at no time is any patient-identifiable information provided to Pharming Healthcare, Inc. Also, for tests involving patients covered under governmental health insurance programs such as Medicare and Medicaid, no information regarding the healthcare professional ordering the test is provided to Pharming Healthcare, Inc. Genetic testing and counseling are available in the US, Canada, and Puerto Rico only. Healthcare professionals and patients who participate in the genetic testing program have no obligation to recommend, purchase, order, prescribe, promote, administer, use, or support any other products or services from the laboratory or from third parties or commercial organizations. Patients who participate in the genetic testing program are free to use the information received through the program to inform their physician and their own healthcare decision-making in any way they deem appropriate.






